Antibody-Drug Conjugates (ADC) Market to Reach USD 30.42 Billion by 2033 | DataM Intelligence
Antibody-Drug Conjugates (ADC) Market- Driven by Advanced Payload Technologies and Strategic Industry Partnerships
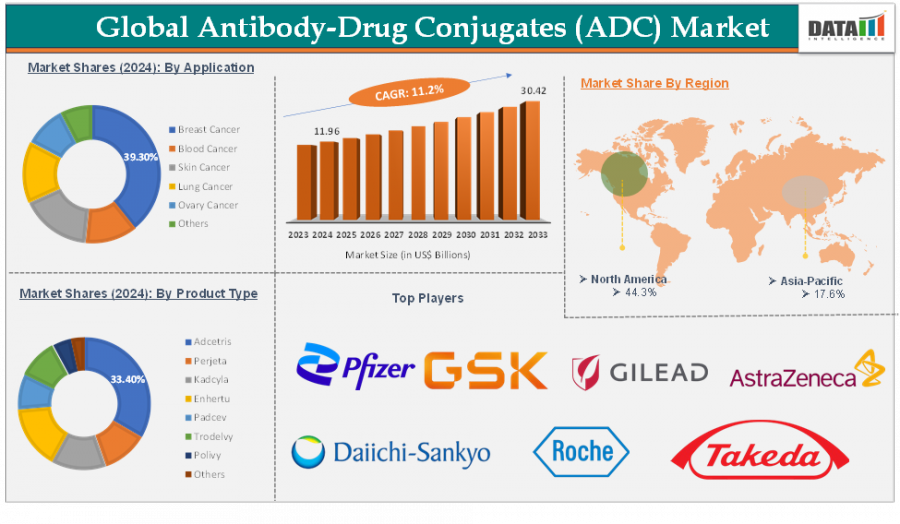
CALIFORNIA, CA, UNITED STATES, July 4, 2025 /EINPresswire.com/ -- The global Antibody-Drug Conjugates (ADC) market is anticipated to experience substantial growth, with a compound annual growth rate (CAGR) of 11.2% from 2025 to 2033, as per DataM Intelligence. The market is projected to reach USD 30.42 billion by 2033. Continuous innovation in linker-payload technologies, expanding clinical applications in oncology, and an increase in strategic collaborations and acquisitions—particularly across key regions such as the United States and Japan—are the primary drivers of this growth.
Download Latest Edition Insights sample report: https://www.datamintelligence.com/download-sample/antibody-drug-conjugates-market
United States and Japan Lead in ADC Innovation and Investment
The United States continues to be at the forefront of ADC development and commercialization in 2025. The FDA granted Priority Review status to datopotamab deruxtecan, a next-generation ADC that targets TROP2, in January 2025. This drug was jointly developed by Daiichi Sankyo and AstraZeneca. This represents a considerable advancement in the treatment of HER2-negative and HER2-low breast cancer, a therapeutic area that is expanding and has substantial unmet requirements.
ADC Therapeutics announced a USD 100 million private funding round in June 2025, thereby securing its operational horizon until at least 2028. This action is indicative of the ongoing investor confidence in ADC platforms for solid and hematologic malignancies.
In the interim, Japan is rapidly becoming a significant innovation base. Taiho Pharmaceutical acquired Swiss-based Araris Biotech in March 2025 in a transaction valued at up to USD 1.14 billion. This acquisition granted Taiho Pharmaceutical access to proprietary linker technology. TIVDAK, which was developed by Seagen and Genmab, was approved by Japan's PMDA in April 2025 for the treatment of advanced cervical cancer. This approval underscores Japan's increasing regulatory support for innovative oncology treatments.
Key Trends Shaping the ADC Market in 2025
In 2025, the ADC market is undergoing a transformation from first-generation molecules to more sophisticated, modular platforms. New developments are focused on the enhancement of efficacy and safety profiles through site-specific conjugation, dual-payload ADCs, and enhanced drug-to-antibody ratios (DARs).
The development of next-generation payloads, including exatecan derivatives and other DNA-damaging compounds, has resulted in a perceptible shift toward deeper tumor penetration and increased cytotoxicity. Companies are also emphasizing ADCs with collateral effects, which facilitate the treatment of tumors with heterogeneous antigen expression. This development is assisting in the surmounting of resistance in malignancies that were previously challenging to treat.
Speak to Our Analyst and Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/antibody-drug-conjugates-market
Company Landscape: Leading Players and Strategic Activity
Through strategic alliances, acquisitions, and product innovation, major corporations continue to fortify their market positions. Enhertu generated over USD 3.75 billion in global sales in 2024, while Daiichi Sankyo and AstraZeneca maintain market leadership. It is anticipated that their pipeline asset, datopotamab deruxtecan, will become another juggernaut, particularly as clinical trials expand into HER3-positive lung cancer.
Seagen, which has been entirely incorporated into Pfizer, is utilizing its combined research capabilities to accelerate the global rollout of TIVDAK and to fortify its position in urothelial and cervical cancers. ImmunoGen, which was acquired by AbbVie, is currently in the process of advancing Elahere, a drug that was approved by the FDA in late 2024 for platinum-resistant ovarian cancer. It is anticipated that Elahere will generate USD 703 million in 2025.
Blenrep is also resuming clinical trials after addressing regulatory concerns, as GSK is reentering the ADC competition. The organization is currently concentrating on the development of multidrug regimens for multiple myeloma. Taiho's acquisition of Araris and the domestic sanction of TIVDAK in Japan are indicative of a more extensive government-supported initiative to position Japan as a regional ADC leader.
Market Segmentation: Oncology Dominance with Expanding Indications
In 2025, oncology will continue to be the primary application for ADCs, with over 90% of the market share. ADCs are predominantly employed in the treatment of breast cancer, non-small cell lung cancer (NSCLC), lymphomas, and ovarian cancer. The efficacy of HER2-low and TROP2-targeted therapies is facilitating the development of ADCs to treat additional challenging malignancies.
Late-stage development is currently underway for emerging targets, including BCMA and HER3. A broader therapeutic applicability is anticipated in the near future, as additional trials are currently underway in gastric, bladder, and prostate malignancies. Although early research is investigating ADCs in infectious and autoimmune diseases, oncology remains the primary commercial and clinical focus.
Gain expert insights on market trends, challenges, and future outlook. Buy the Full Report Now and strengthen your strategy with DataM Intelligence: https://www.datamintelligence.com/buy-now-page?report=antibody-drug-conjugates-market
Regional Analysis: United States Leads, Japan Expands Rapidly
In 2024, the United States accounted for over 60% of global revenue in the ADC market, maintaining its dominant position. FDA regulatory support, sophisticated biotech communities, and expanding contract manufacturing capacity are the primary factors contributing to this dominance. Cities such as Boston and San Diego continue to be hubs for ADC innovation, as they are home to significant CDMOs and specialized payload facilities.
Japan is the fastest-growing regional market, bolstered by a growing number of clinical trials, strong local competitors such as Daiichi Sankyo and Taiho, and favorable PMDA guidelines. The establishment of ADC manufacturing ecosystems, which include cleanroom infrastructure and cytotoxic handling capabilities, is being facilitated by government-backed initiatives, which have positioned Japan as a significant regional participant in the Asia-Pacific region.
DataM Intelligence Viewpoint
The ADC market is seeing rapid expansion, driven by scientific advances and commercial mergers. As global competition heats up, success will be determined by technical innovation, regulatory agility, and manufacturing scalability, according to DataM Intelligence's Oncology Research Team. "With a predicted market size of USD 30.42 billion by 2033, stakeholders should prioritize investments in modular ADC platforms, linker-payload engineering, and cross-border collaborations, particularly in the United States and Japan.
Unlock 360° Market Intelligence with DataM Subscription Services:
https://www.datamintelligence.com/reports-subscription
✅ Technology Roadmap Analysis
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Pipeline Analysis For Drugs Discovery
✅ Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Competitive Landscape
Have a look at our Subscription Dashboard:
https://www.youtube.com/watch?v=x5oEiqEqTWg
Related Reports:
Biomarkers market is set to reach US$ 148.88 billion by 2032
Cancer Diagnostics Market is Set to Reach US$ 42.64 Billion by 2033
Sai Kumar
DataM Intelligence 4market Research LLP
+1 877-441-4866
sai.k@datamintelligence.com
Visit us on social media:
LinkedIn
X
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release