Toxicity Testing Outsourcing Market to Reach USD 8.28 Billion by 2032 | SNS Insider
Market poised for a 9.2% CAGR growth, driven by rising R&D investments and regulatory compliance needs.
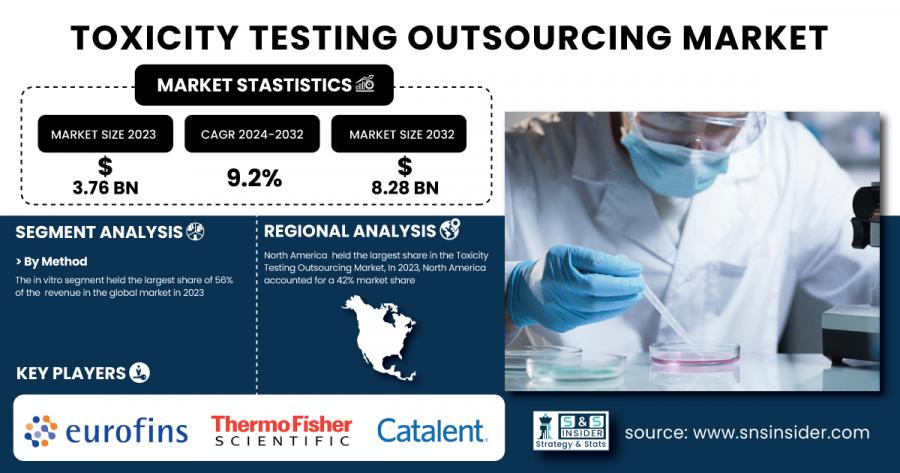
AUSTIN, TX, UNITED STATES, February 25, 2025 /EINPresswire.com/ -- According to a recent report by SNS Insider, the Toxicity Testing Outsourcing Market is set to witness remarkable growth, expanding from USD 3.76 billion in 2023 to USD 8.28 billion by 2032, at a CAGR of 9.2% during the forecast period of 2024-2032. The increasing demand for toxicity testing in pharmaceuticals, chemicals, and cosmetics, coupled with stringent regulatory standards, is driving the market’s expansion.
Market analysis
The increasing focus on product safety and regulatory compliance regulations is driving the growth of toxicity testing outsourcing market. Regulations around the globe are tightening up the safety standards requiring extensive assessments of the toxicity of pharmaceuticals, chemicals, and consumer products. Testing protocols have become increasingly complex, and as a result, demand for specialized expertise has led to a boom in outsourcing to contract research organizations (CROs). Moreover, the increasing utilization of alternative testing processes, like in vitro and in silico models, to minimize the need for animal testing is also driving the market growth. Recent government statistics highlight the importance of the toxicity testing process as a public health measure. For example, the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have instituted stringent toxicity testing requirements for drug approval, creating significant outsourcing services demand. Moreover, with the increased emphasis on environmental safety, as seen in the EU’s REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) program, the demand for toxicity testing in the chemical industry has also grown.
Get a Free Sample Report@ https://www.snsinsider.com/sample-request/5771
Market Segmentation
By Method
In 2023, the toxicity testing outsourcing market was led by the in vitro segment with nearly 56% revenue share. Cell-based models (in vitro) are the most preferred and widely used methods and are more economically friendly, reproducible, and in line with the international movement towards reducing animal testing. Such approaches can be extremely useful for preliminary toxicity screening and high-throughput assays. In vivo testing, which involves animal models, remains essential for certain regulatory requirements but is gradually being supplemented by alternative methods. In silico testing, which uses computer simulations and predictive modeling, is gaining traction due to its ability to provide rapid and cost-effective toxicity assessments.
By GLP Type
In 2023, the GLP (Good Laboratory Practice) segment held the largest share of the market. GLP-compliant testing is mandatory for regulatory submissions, ensuring the reliability and integrity of toxicity data. Non-GLP testing, though less rigorous, is commonly performed for exploratory research and early-phase screening. This segment is witnessing growth due to the rising need for GLP-compliant testing services, particularly in the pharmaceutical and chemical industries. GLP accreditation is increasingly in demand among CROs for data generation, highlighting the need for high-quality data that meets regulatory compliance standards.
By Application
In 2023, pharmaceutical and biopharmaceutical held the largest market share of the revenue of 62%. Testing for toxicity is an important step in drug development, which guarantees the safety and efficacy of new therapeutics. As the pipeline of biologics and complex drugs further expands, it has deepened the demand for specialized toxicity testing services. The chemical and consumer products segments are also large contributors in terms of the volume of submissions they submit for safety assessments, as required by regulation. Moreover, the agrochemical industry has increasingly promoted outsourcing of toxicity testing.
By End Use
The largest end-users in 2023 were pharmaceutical and biopharmaceutical companies, driven by increasing demand for complete toxicity testing during drug development. Also significant are contract research organizations (CROs), which provide specialized testing services to numerous industries. Driven by the need for specialized expertise and cutting-edge technologies, academic and research institutions are outsourcing toxicity testing to CROs. Another important end-user of toxicity testing is the chemical industry, which seeks to comply with regulatory standards and ensure the safety of its products.
Speak with Our Expert Analyst Today to Gain Deeper Insights @ https://www.snsinsider.com/request-analyst/5771
Regional Analysis
The largest share of the toxicity testing outsourcing market in 2023 was held by North America, which accounted for 42% of the total revenue. The region accounts for the leading share owing to its established pharmaceutical and biotechnology industries, strict regulatory environment and high adoption of advanced testing technologies. The market is also further driven by the presence of well-known CROs, alongside an advanced healthcare infrastructure. Asia-Pacific is expected to record the fastest growth during the forecast period, with the rise in investments in healthcare infrastructure, increase in pharmaceutical R&D activities, and increase in product safety awareness being the key factors
Key Players in Toxicity Testing Outsourcing Market
• Eurofins Scientific (In Vitro Toxicology Services, DiscoveryAI)
• SGS SA (Preclinical Safety Testing, In Vitro Toxicology Testing)
• Charles River Laboratories (Safety Assessment Services, In Vivo Toxicology Studies)
• Thermo Fisher Scientific, Inc. (Cell-Based Assays, ADME-Tox Services)
• Intertek Group plc (Regulatory Toxicology Services, Ecotoxicology Testing)
• Catalent, Inc. (Preclinical Toxicology Testing, Drug Metabolism Studies)
• ICON plc (Nonclinical Safety Assessment, Toxicokinetic Studies)
• Medpace (Preclinical Toxicology Services, Safety Pharmacology)
• WuXi AppTec (In Vitro Toxicity Testing, In Vivo Toxicology Services)
• Labcorp Drug Development (Preclinical Toxicology Services, Genetic Toxicology Testing)
• Element Materials Technology (Materials Testing Services, Certification Schemes)
• IQVIA (Clinical Trial Execution Services, Data Management Solutions)
• Covance Inc. (Safety Assessment Services, Toxicology Testing)
• Envigo (Nonclinical Safety Assessment, Reproductive Toxicology)
• Toxikon Corporation (Extractables and Leachables Testing, Biocompatibility Testing)
• MB Research Laboratories (In Vitro Toxicology Testing, Dermal Irritation Studies)
• Charles River Laboratories (Safety Assessment Services, In Vivo Toxicology Studies)
• Pharmaron (Preclinical Safety Assessment, Genetic Toxicology)
• PRA Health Sciences (Clinical Development Services, Toxicology Testing)
• Syneos Health (Early Phase Development, Safety Assessment Services)
Recent Developments
• In 2023, Charles River Laboratories expanded its toxicity testing capabilities by introducing advanced in vitro platforms for high-throughput screening.
• In January 2024, Eurofins Scientific received regulatory approval for its new in silico toxicity prediction tool, designed to enhance the efficiency of early-stage drug development.
Buy Full Research Report on Toxicity Testing Outsourcing Market 2024-2032 @ https://www.snsinsider.com/checkout/5771
About Us:
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
Jagney Dave
SNS Insider Pvt. Ltd
+1 315 636 4242
email us here
Visit us on social media:
Facebook
X
LinkedIn
Instagram
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release